Chemistry, 27.01.2020 21:31 reeeeeee32
Binary compound created by reaction of an unknown element e and sulfur contains 27.52% e and 72.48% s by mass. if the formula of the compound is e4s10, calculate the atomic mass of e.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
Binary compound created by reaction of an unknown element e and sulfur contains 27.52% e and 72.48%...
Questions




Mathematics, 18.05.2021 19:20


Biology, 18.05.2021 19:20




Computers and Technology, 18.05.2021 19:20










Mathematics, 18.05.2021 19:20

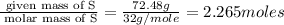
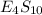
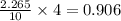 moles of E
moles of E