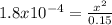Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
Calculate the ph for the following weak acid.
a solution of hcooh has 0.15m hcooh at equ...
a solution of hcooh has 0.15m hcooh at equ...
Questions

Mathematics, 20.07.2020 08:01




Mathematics, 20.07.2020 08:01


History, 20.07.2020 08:01



Biology, 20.07.2020 08:01


Mathematics, 20.07.2020 08:01



Biology, 20.07.2020 08:01




Mathematics, 20.07.2020 08:01

Mathematics, 20.07.2020 08:01

![ka = \frac{[H3O][HCOO]}{[HCOOH]}](/tpl/images/0471/8959/57b44.png)
![1.8 x 10^{-4} = \frac{[x][x]}{0.15 - x}](/tpl/images/0471/8959/c09c0.png)