
Chemistry, 27.01.2020 01:31 eagles2286
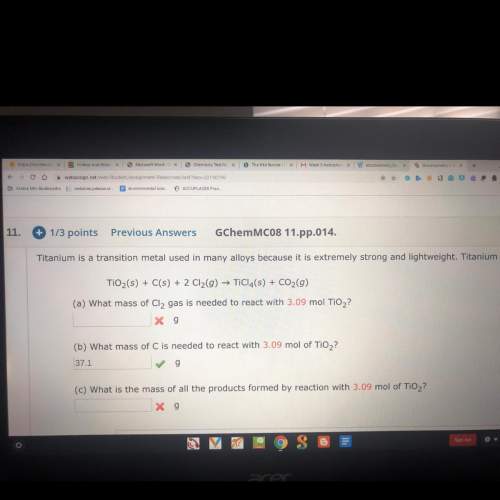
Titanium is a transition metal used in many alloys because it is extremely strong and lightweight. titanium tetrachloride (ticl4) is extracted from titanium oxide using chloride and coke (carbon).
a) what mass of cl2 gas is needed to react with 3.09 mol tio2?
c) what is the mass of all the products formed by reaction with 3.09 mol tio2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

You know the right answer?
Titanium is a transition metal used in many alloys because it is extremely strong and lightweight. t...
Questions



English, 26.01.2022 14:00



SAT, 26.01.2022 14:00


Mathematics, 26.01.2022 14:00



Physics, 26.01.2022 14:00



Mathematics, 26.01.2022 14:00


Computers and Technology, 26.01.2022 14:00


Mathematics, 26.01.2022 14:00




