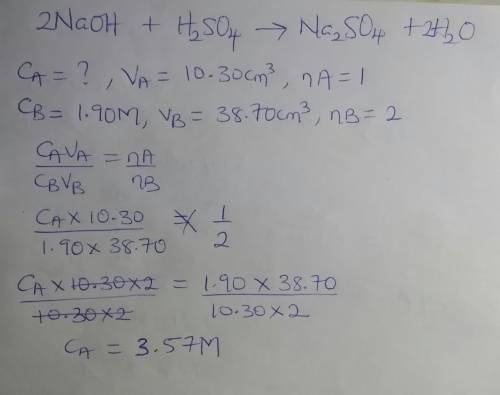Chemistry, 27.01.2020 00:31 estefaniapenalo
If it takes 38.70cm³ of 1.90 m naoh to neutralize 10.30cm³ of h2so4 in a battery, what's the molarity of the h2so4 ?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
If it takes 38.70cm³ of 1.90 m naoh to neutralize 10.30cm³ of h2so4 in a battery, what's the molarit...
Questions



English, 24.09.2019 18:00

Mathematics, 24.09.2019 18:00

History, 24.09.2019 18:00

Mathematics, 24.09.2019 18:00




Social Studies, 24.09.2019 18:00

Mathematics, 24.09.2019 18:00

Mathematics, 24.09.2019 18:00



Chemistry, 24.09.2019 18:10

Biology, 24.09.2019 18:10

Mathematics, 24.09.2019 18:10



English, 24.09.2019 18:10