
Chemistry, 25.01.2020 06:31 jaleewoodyard1
Apharmacist–herbalist mixed 100 g lots o st. john’s wort containing the ollowing percentages o the active component hypericin: 0.3%, 0.7%, and 0.25%. calculate the percent strength o hypericin in the mixture.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 23.06.2019 11:40
An electron moved from a lower energy level to a higher energy level. what most likely happened during the transition? a random amount of light was released. a fixed amount of energy was absorbed. a fixed amount of energy was released. a random amount of light was absorbed.
Answers: 1


Chemistry, 23.06.2019 14:00
Ineed in question 5and6 in science the subject is science
Answers: 1
You know the right answer?
Apharmacist–herbalist mixed 100 g lots o st. john’s wort containing the ollowing percentages o the a...
Questions

Mathematics, 12.12.2021 22:50




Mathematics, 12.12.2021 22:50

Social Studies, 12.12.2021 22:50

Biology, 12.12.2021 22:50


Mathematics, 12.12.2021 22:50




Mathematics, 12.12.2021 22:50

Chemistry, 12.12.2021 22:50

Mathematics, 12.12.2021 22:50



Mathematics, 12.12.2021 22:50


Mathematics, 12.12.2021 22:50

 × 100
× 100 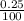 × 100
× 100 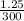 × 100
× 100 

