

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
Are the bonds in each of the following substances ionic, nonpolar covalent, or polar covalent?
Questions

Chemistry, 13.01.2020 20:31

Mathematics, 13.01.2020 20:31


English, 13.01.2020 20:31









Social Studies, 13.01.2020 20:31


Chemistry, 13.01.2020 20:31

Mathematics, 13.01.2020 20:31




Mathematics, 13.01.2020 20:31

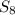 non polar covalent ( because each sulphur atom shares electron and the net dipole moment is zero.)
non polar covalent ( because each sulphur atom shares electron and the net dipole moment is zero.)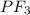 polar covalent ( because of triagonal pyramidal geometry and strong electronegativity of F atom it is polar and covalency is due to the share of electron with each F atom.)
polar covalent ( because of triagonal pyramidal geometry and strong electronegativity of F atom it is polar and covalency is due to the share of electron with each F atom.)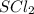 polar covalent ( because of tetrahedral geometry and sharing of electrons between S and Cl.)
polar covalent ( because of tetrahedral geometry and sharing of electrons between S and Cl.)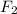 non polar covalent ( because of linear geometry they are non polar and bond between them is formed due to sharing electrons.)
non polar covalent ( because of linear geometry they are non polar and bond between them is formed due to sharing electrons.)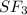 polar covalent ( because of T- shape geometry they are non polar and due to sharing of electrons they are polar covalent.)
polar covalent ( because of T- shape geometry they are non polar and due to sharing of electrons they are polar covalent.)

