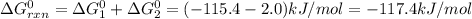Given the reactions below, answer the following questions.
cl_2(g) + f_2(g) rlhar 2clf(g) del...

Chemistry, 25.01.2020 04:31 tsmalls70988
Given the reactions below, answer the following questions.
cl_2(g) + f_2(g) rlhar 2clf(g) delta g degree_rxn = 115.4 kj/mol
cl_2(g) + br_2(g) rlhar 2clbr(g) delta g degree_rxn = -2.0 kj/mol
calculate the delta g degree_rxn for 2clf(g) + br_2(g) rlhar 2clbr(g) + f_2(g) kj/mol

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

You know the right answer?
Questions

Mathematics, 27.10.2020 20:30

History, 27.10.2020 20:30


Mathematics, 27.10.2020 20:30

Mathematics, 27.10.2020 20:30

History, 27.10.2020 20:30

English, 27.10.2020 20:30

Social Studies, 27.10.2020 20:30

Biology, 27.10.2020 20:30





Arts, 27.10.2020 20:30

English, 27.10.2020 20:30



SAT, 27.10.2020 20:30


Mathematics, 27.10.2020 20:30

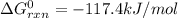
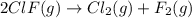 ;
; 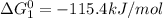
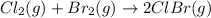 ;
; 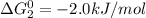
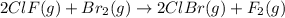 ;
;