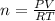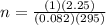Chemistry, 25.01.2020 02:31 carbpotatoes
Yeast and other organisms can convert glucose (c6h12o6) to ethanol (ch3ch2oh) through a process called alcoholic fermentation. the net reaction is:
(l) +2co2(g)
calculate the mass of glucose required to produce 2.25l of co2 measured at p=1 atm and t=295k

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
Yeast and other organisms can convert glucose (c6h12o6) to ethanol (ch3ch2oh) through a process call...
Questions

Social Studies, 18.03.2021 01:40




Mathematics, 18.03.2021 01:40


Mathematics, 18.03.2021 01:40




Physics, 18.03.2021 01:40

History, 18.03.2021 01:40


Mathematics, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40




Mathematics, 18.03.2021 01:40