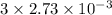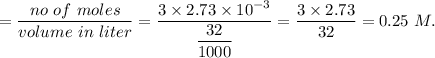Chemistry, 25.01.2020 02:31 Ayyyyeeeeeeewuzgud
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivalence point is reached when 24.83 ml of naoh solution is added. what is the concentration of the unknown h3po4 solution? the neutralization reaction is
h3po4(aq)+3naoh(aq)→3h2o(l)+na3po4( aq)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1


You know the right answer?
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivale...
Questions





English, 22.05.2021 23:40

Mathematics, 22.05.2021 23:40




Mathematics, 22.05.2021 23:50

History, 22.05.2021 23:50


Engineering, 22.05.2021 23:50


Arts, 22.05.2021 23:50

English, 22.05.2021 23:50


Social Studies, 22.05.2021 23:50


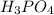 in sample is 0.25 M.
in sample is 0.25 M.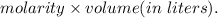
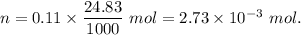
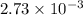 mol of NaOH reacts with
mol of NaOH reacts with