
Chemistry, 25.01.2020 01:31 princeofpowerjr
Awater treatment plant receives the source water with an average ca2+ concentration of 46.9 mg/l and mg2+ concentration of 14.8 mg/l. the plant is treating 80 million gallons of water per day. what mass of solids will be produced per day if all of the calcium and magnesium are converted to caco3(s) and mg(oh)2(s) in the softening process? give your answer in kg.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3
You know the right answer?
Awater treatment plant receives the source water with an average ca2+ concentration of 46.9 mg/l and...
Questions

Business, 09.07.2019 16:00

English, 09.07.2019 16:00

Social Studies, 09.07.2019 16:00

History, 09.07.2019 16:00

English, 09.07.2019 16:00

Computers and Technology, 09.07.2019 16:00

Biology, 09.07.2019 16:00

Computers and Technology, 09.07.2019 16:00


Health, 09.07.2019 16:00

Computers and Technology, 09.07.2019 16:00


Mathematics, 09.07.2019 16:00

Biology, 09.07.2019 16:00

Computers and Technology, 09.07.2019 16:00




Computers and Technology, 09.07.2019 16:00

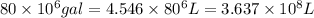
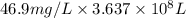
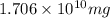
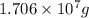
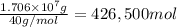
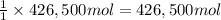 of calcium carbonate
of calcium carbonate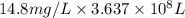
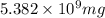
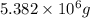
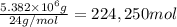
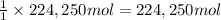 of magnesium hydroxide
of magnesium hydroxide

