Consider the reaction:
2al(s) + fe2o3(s) - al2o3(s) + 2fe(s)
the ah, for fe2o3(s) = -82...

Chemistry, 24.01.2020 23:31 SignoraPenguino
Consider the reaction:
2al(s) + fe2o3(s) - al2o3(s) + 2fe(s)
the ah, for fe2o3(s) = -824.3 kj/mole. the ah, for al2o3(s) = -1675.7 kj/mole.
finish the equation.
ahxn = [(1)
c
y
kj/mole) + (2)
y kj/mole)] - [(1)
kj/mole) + (2) (
kj/mole)]

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Questions







Mathematics, 09.10.2019 17:20


Computers and Technology, 09.10.2019 17:20

Mathematics, 09.10.2019 17:20

Mathematics, 09.10.2019 17:20





History, 09.10.2019 17:20

Mathematics, 09.10.2019 17:20




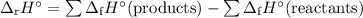
![\begin{array}{rcl}\Delta_{\text{r}}H^{\circ} & = & [1(-1675.7) + 2(0)] - [2(0) - 1(-824.3)]\\& = & -1675.7 + 824.3\\& = & \textbf{-851.4 kJ/mol}\\\end{array}\\\text{The enthalpy change is } \large \boxed{\textbf{-851.4 kJ/mol}}](/tpl/images/0469/5682/d5fae.png)


