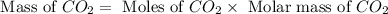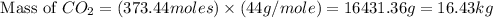Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
What mass of co2 is produced from the combustion of 2.00 gallons of gas? assume the gas is all octa...
Questions


Mathematics, 07.04.2020 20:56

Mathematics, 07.04.2020 20:56

Mathematics, 07.04.2020 20:57

English, 07.04.2020 20:57













Mathematics, 07.04.2020 20:57


History, 07.04.2020 20:57

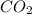 produced form the combustion is, 16.43 kg
produced form the combustion is, 16.43 kg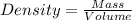
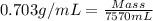
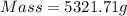
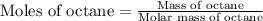
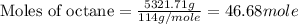
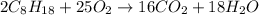
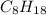 react to give 16 moles of
react to give 16 moles of 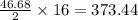 moles of
moles of