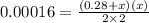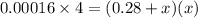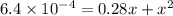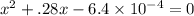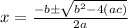Chemistry, 24.01.2020 10:31 matthewquattlebum
When solid nh4hs and 0.28 mol nh3(g) were placed in a 2 l vessel at 24◦c, the equilibriumnh4hs(s)⇀↽nh3(g) + h2s(g)for which kc= 0.00016, was reached. what is the equilibrium concentration of nh3? answer in units of mol/l

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
When solid nh4hs and 0.28 mol nh3(g) were placed in a 2 l vessel at 24◦c, the equilibriumnh4hs(s)⇀↽n...
Questions



History, 18.05.2021 23:50


Mathematics, 18.05.2021 23:50


Mathematics, 18.05.2021 23:50

Biology, 18.05.2021 23:50




Mathematics, 18.05.2021 23:50


English, 18.05.2021 23:50





Mathematics, 18.05.2021 23:50

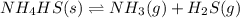
![Kc=[NH_{3}][H_{2}S]](/tpl/images/0468/7808/2a772.png)
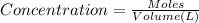
![[NH_{3}]=\frac{0.28 +x}{2}](/tpl/images/0468/7808/aa8ed.png)
![[H_{2}S]=\frac{x}{2}](/tpl/images/0468/7808/ba461.png)