Chemistry, 24.01.2020 03:31 oranjejuice
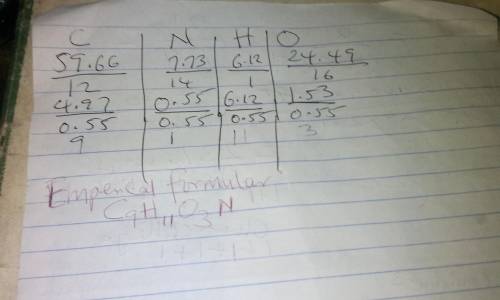
The compound tyrosine contains 59.66% c, 6.12% h, 7.73% n, and 26.49% o by mass. what is the empirical formula of tyrosine?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2
You know the right answer?
The compound tyrosine contains 59.66% c, 6.12% h, 7.73% n, and 26.49% o by mass. what is the empiric...
Questions

Mathematics, 03.06.2020 19:00

Mathematics, 03.06.2020 19:00

History, 03.06.2020 19:00

Spanish, 03.06.2020 19:00

Mathematics, 03.06.2020 19:00

History, 03.06.2020 19:00


Mathematics, 03.06.2020 19:00




Mathematics, 03.06.2020 19:00

Mathematics, 03.06.2020 19:00

Mathematics, 03.06.2020 19:00


Mathematics, 03.06.2020 19:00

Mathematics, 03.06.2020 19:00

Mathematics, 03.06.2020 19:00

Mathematics, 03.06.2020 19:00

Physics, 03.06.2020 19:00