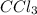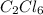Chemistry, 24.01.2020 02:31 hqlego6882
Acompound contains 10.13% c and 89.87% cl (by mass). determine both the empirical formula and the molecular formula of the compound given that the molar mass is 237 g/mol.
ccl3
c2cl
ccl

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

You know the right answer?
Acompound contains 10.13% c and 89.87% cl (by mass). determine both the empirical formula and the mo...
Questions













World Languages, 04.08.2021 17:40

Mathematics, 04.08.2021 17:40



Mathematics, 04.08.2021 17:40

Mathematics, 04.08.2021 17:40

Mathematics, 04.08.2021 17:40