
Chemistry, 24.01.2020 02:31 SkirrtCrackers
For a certain chemical reaction, the standard gibbs free energy of reaction is 144. kj. calculate the temperature at which the equilibrium constant k = 5.9 × 10 . round your answer to the nearest degree.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
For a certain chemical reaction, the standard gibbs free energy of reaction is 144. kj. calculate th...
Questions



Mathematics, 11.05.2021 16:10



History, 11.05.2021 16:10





Mathematics, 11.05.2021 16:10




Mathematics, 11.05.2021 16:10


Social Studies, 11.05.2021 16:10

English, 11.05.2021 16:10

Mathematics, 11.05.2021 16:10

Mathematics, 11.05.2021 16:10

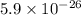 .
.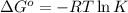
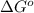 = standard Gibbs free energy = 144.0 kJ=144,000 J (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = 144.0 kJ=144,000 J (Conversion factor: 1kJ = 1000J)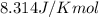
![144,000 J/mol=-(8.3145J/Kmol)\times T\times \ln [5.9\times 10^{-26}]](/tpl/images/0468/2071/84a5d.png)
![T=\frac{144,000 J/mol}{-(8.314 J/Kmol)\times \ln [5.9\times 10^{-26}]}](/tpl/images/0468/2071/493d4.png)


