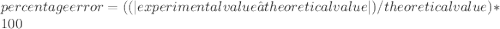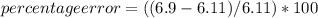Chemistry, 24.01.2020 00:31 GhostBoooty
In a laboratory activity, the density of a sample of vanadium is determined to be 6.9 g/cm3 at room temperature. what is the percent error for the determined value?
a. 0.15%
b. 0.87%
c. 13%
d. 15%

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1
You know the right answer?
In a laboratory activity, the density of a sample of vanadium is determined to be 6.9 g/cm3 at room...
Questions






Social Studies, 16.03.2020 20:02

History, 16.03.2020 20:02

History, 16.03.2020 20:02


English, 16.03.2020 20:03



Health, 16.03.2020 20:03



Social Studies, 16.03.2020 20:03




Physics, 16.03.2020 20:03