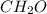a 0.1014 g sample of a purified cho compound was burned in a combustion apparatus and produced 0.1486 g co2 and 0.0609 g of h2o. mass spectrometry analysis revealed that the cho compound had a molar mass of 180 g/mol. what is the molecular formula for this compound?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
a 0.1014 g sample of a purified cho compound was burned in a combustion apparatus and produced 0.148...
Questions










Mathematics, 21.02.2020 19:54

Biology, 21.02.2020 19:54


English, 21.02.2020 19:54




English, 21.02.2020 19:54

Mathematics, 21.02.2020 19:54


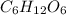
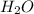 = 0.0609 g /18 g/mol = 0.00338 moles
= 0.0609 g /18 g/mol = 0.00338 moles
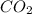 = 0.1486 g /44.01 g/mol = 0.00337 moles
= 0.1486 g /44.01 g/mol = 0.00337 moles