
Chemistry, 23.01.2020 22:31 ashtonlauber95
Suppose 0.10 mol of cu(no_3)_2 and 1.50 mol of nh_3 are dissolved in water and diluted to a total volume of 1.00 l. calculate the concentrations of cu(nh_3)_4^2+ and of cu^2+ at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Suppose 0.10 mol of cu(no_3)_2 and 1.50 mol of nh_3 are dissolved in water and diluted to a total vo...
Questions



English, 20.08.2020 17:01

Computers and Technology, 20.08.2020 17:01

Mathematics, 20.08.2020 17:01

Mathematics, 20.08.2020 17:01


Geography, 20.08.2020 17:01

Mathematics, 20.08.2020 17:01

Health, 20.08.2020 17:01

Mathematics, 20.08.2020 17:01

Social Studies, 20.08.2020 17:01






Chemistry, 20.08.2020 17:01

Arts, 20.08.2020 17:01

Spanish, 20.08.2020 17:01

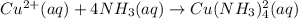
![K_{f} = \frac{[Cu(NH3)^{2+}_{4}]}{[Cu^{2+}][NH_{3}]_{4}}](/tpl/images/0467/9077/53418.png)
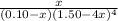
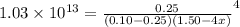
![[NH_{3}] = 1.50 - 4x = (\frac{2.33}{1.03 \times 10^{13}})^{\frac{1}{4}](/tpl/images/0467/9077/925c0.png)
![[Cu(NH_{3})^{2+}_{4}]](/tpl/images/0467/9077/dbe3c.png)
![\frac{1.50 - 2.31284 \times 10{-4}}{4}]](/tpl/images/0467/9077/19dce.png)
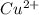 ) is 0.37491425 M.
) is 0.37491425 M.

