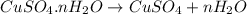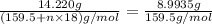Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hydrate is heated to a high enough temperature, h 2 o ( g ) can be driven off, leaving the grey‑white anhydrous salt cuso 4 ( s ) . a 14.220 g sample of the hydrate was heated to 300 ∘ c . the resulting cuso 4 ( s ) had a mass of 8.9935 g . calculate the val

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hyd...
Questions

Health, 29.06.2019 23:00





Biology, 29.06.2019 23:00




Arts, 29.06.2019 23:00



History, 29.06.2019 23:00




Mathematics, 29.06.2019 23:00

Physics, 29.06.2019 23:00

Mathematics, 29.06.2019 23:00

Biology, 29.06.2019 23:00

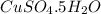 .
.