Hcl(aq)+naoh(aq)→nacl(aq)+h2o(l)δh° =−57.1kj/molrxn
the chemical equation above represents th...

Chemistry, 23.01.2020 20:31 powellkolbie
Hcl(aq)+naoh(aq)→nacl(aq)+h2o(l)δh° =−57.1kj/molrxn
the chemical equation above represents the reaction between hcl(aq) and naoh(aq). when equal volumes of 1.00mhcl(aq) and 1.00mnaoh(aq) are mixed, 57.1kj of heat is released. if the experiment is repeated with 2.00mhcl(aq), how much heat would be released?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1


Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Questions


History, 30.09.2020 04:01

Mathematics, 30.09.2020 04:01

Mathematics, 30.09.2020 04:01

History, 30.09.2020 04:01

English, 30.09.2020 04:01



Mathematics, 30.09.2020 04:01

Mathematics, 30.09.2020 04:01

History, 30.09.2020 04:01

Mathematics, 30.09.2020 04:01

English, 30.09.2020 04:01






Mathematics, 30.09.2020 04:01

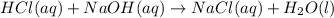 ΔH°=-57.1kJ/mol
ΔH°=-57.1kJ/mol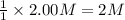 of NaOH
of NaOH


