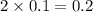Chemistry, 23.01.2020 18:31 danielacortevpe3i66
Raoult's law accounts for the fact that the vapor pressure of a solvent will decrease as the mole fraction of the solvent is decreased. in considering the mole fraction, it is important to consider the total moles of dissolved particles. remember: a particle can be a dissolved molecule or ion. which aqueous solutions would have the lowest vapor pressure

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
Raoult's law accounts for the fact that the vapor pressure of a solvent will decrease as the mole fr...
Questions

Mathematics, 13.01.2021 23:50



Mathematics, 13.01.2021 23:50



Mathematics, 13.01.2021 23:50






Physics, 13.01.2021 23:50


Mathematics, 13.01.2021 23:50



Mathematics, 13.01.2021 23:50

Arts, 13.01.2021 23:50

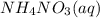 , 0.1 M
, 0.1 M 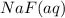 , 0.1 M
, 0.1 M 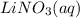 , 0.1 M
, 0.1 M 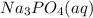 and 0.1 M
and 0.1 M 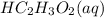
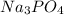
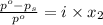
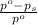 = relative lowering in vapor pressure
= relative lowering in vapor pressure
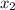 = mole fraction of solute
= mole fraction of solute 
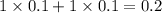
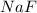
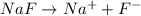
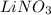
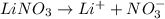
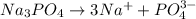
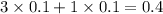
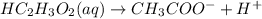 [/tex]
[/tex]