Chemistry, 23.01.2020 05:31 lraesingleton
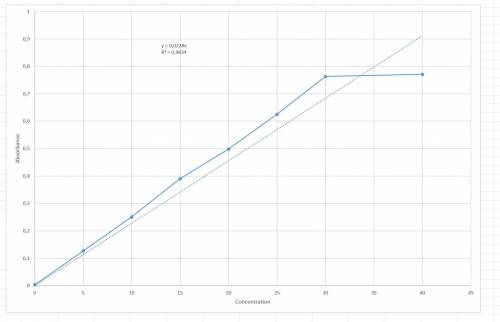
The following results were obtained when each of a series of standard silver solutions was analyzed by flame-atomic absorption spectrometry: concentration on 0 5 10 15 20 25 30 40 (ng. ml1) absorbance(r. u.) 0.003 0.127 0.251 0.390 0.498 0.625 0.763 0.771 determine the slope and intercept of the calibration plot, along with their confidence limits (95%). using the data from exercise 1 above, estimate the confidence limits for the silver concentration in: a) a sample giving an absorbance of 0.456 in a single determination. b) a sample giving absorbance values of 0.308, 0.317, 0.347, and 0.412 in four separate determinations. graph: a straight line excel plot is shown in the question

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3
You know the right answer?
The following results were obtained when each of a series of standard silver solutions was analyzed...
Questions





Mathematics, 04.10.2019 23:40



World Languages, 04.10.2019 23:40

Biology, 04.10.2019 23:40


Mathematics, 04.10.2019 23:40

Spanish, 04.10.2019 23:40



Mathematics, 04.10.2019 23:40



History, 04.10.2019 23:40