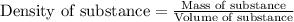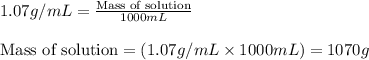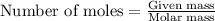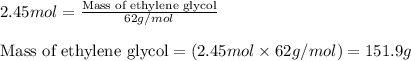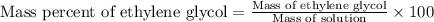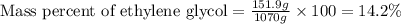Chemistry, 23.01.2020 04:31 akhawajaaa
What is the mass percent of ethylene glycol in a 2.45 m solution of the ethylene glycol in water, the density of the solution is 1.07?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2
You know the right answer?
What is the mass percent of ethylene glycol in a 2.45 m solution of the ethylene glycol in water, th...
Questions

Mathematics, 05.05.2020 14:02


English, 05.05.2020 14:02

Computers and Technology, 05.05.2020 14:02


Mathematics, 05.05.2020 14:02

Chemistry, 05.05.2020 14:02

Spanish, 05.05.2020 14:02

Social Studies, 05.05.2020 14:02

Mathematics, 05.05.2020 14:02


History, 05.05.2020 14:02



History, 05.05.2020 14:02



Mathematics, 05.05.2020 14:02


History, 05.05.2020 14:02