Fe(s) + 2hcl(aq) --> fecl2(aq) + h2(g)
when a student adds 30.0 ml of 1.00 m hcl to...

Chemistry, 23.01.2020 03:31 coollid876
Fe(s) + 2hcl(aq) --> fecl2(aq) + h2(g)
when a student adds 30.0 ml of 1.00 m hcl to 0.56 g of powdered fe, a reaction occurs according to the equation above. when the reaction is complete at 273 k and 1.0 atm, which of the following is true?
a) hcl is in excess, and 0.100 mol of hcl remains unreacted.
d) 0.22 l of h2 has been produced.
the correct answer is d. i can't figure out why a is wrong.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Questions

Mathematics, 20.10.2020 03:01

History, 20.10.2020 03:01



Mathematics, 20.10.2020 03:01




History, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01


Chemistry, 20.10.2020 03:01



Spanish, 20.10.2020 03:01



Social Studies, 20.10.2020 03:01


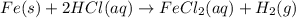
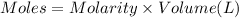
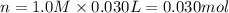
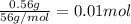
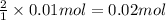 of HCl
of HCl

