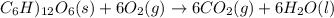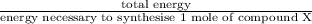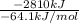Chemistry, 23.01.2020 03:31 coleman310
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and water liberates 2810 kj (δg°\' = –2810 kj/mol). if the energy generated by the combustion of fructose is entirely converted to the synthesis of a hypothetical compound x, calculate the number of moles of the compound that could theoretically be generated. use the value δg°\'compound x = − 64.1 kj/mol kj/mol. round your answer to two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and...
Questions


Mathematics, 02.12.2021 01:00

Social Studies, 02.12.2021 01:00



Mathematics, 02.12.2021 01:00


Biology, 02.12.2021 01:00


Mathematics, 02.12.2021 01:00


Mathematics, 02.12.2021 01:00


History, 02.12.2021 01:00