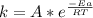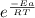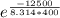Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Calculate the fraction of atoms in a sample of argon gas at 400 k that have an energy of 12.5 kj or...
Questions

Biology, 09.12.2021 01:00

History, 09.12.2021 01:00

English, 09.12.2021 01:00

History, 09.12.2021 01:00



Health, 09.12.2021 01:00


Computers and Technology, 09.12.2021 01:00

Geography, 09.12.2021 01:00





Physics, 09.12.2021 01:00


Mathematics, 09.12.2021 01:00

Mathematics, 09.12.2021 01:00

English, 09.12.2021 01:00

English, 09.12.2021 01:00