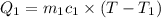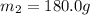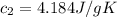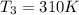Chemistry, 22.01.2020 20:31 justin5163
A75.0 g piece of gold at 650. k is dropped into 180. g of h2o(l) at 310. k in an insulated container at 1 bar pres- sure. calculate the temperature of the system once equilib- rium has been reached. assume that cp, m for au and h2o is constant at their values for 298 k throughout the temperature range of interest.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 11:00
Suppose you increase your walking speed from 7 m/s to 15 m/s in a period of 1 s. what is your acceleration?
Answers: 1
You know the right answer?
A75.0 g piece of gold at 650. k is dropped into 180. g of h2o(l) at 310. k in an insulated container...
Questions

Mathematics, 20.12.2021 04:00

Mathematics, 20.12.2021 04:00

English, 20.12.2021 04:00

Physics, 20.12.2021 04:00

English, 20.12.2021 04:00

Mathematics, 20.12.2021 04:00

Physics, 20.12.2021 04:00

English, 20.12.2021 04:00




Mathematics, 20.12.2021 04:00



Mathematics, 20.12.2021 04:00


English, 20.12.2021 04:00

English, 20.12.2021 04:00


Chemistry, 20.12.2021 04:00

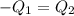
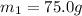
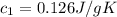
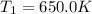
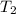 =T
=T