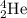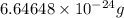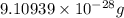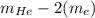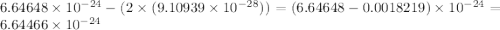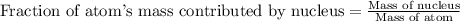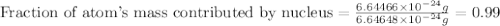Helium is the lightest noble gas and the second most abundant element (after hydrogen) in the universe. the mass of a helium−4 atom is 6.64648 × 10−24g, and each of its two electrons has a mass of 9.10939 × 10−28g. what fraction of this atom's mass is contributed by its nucleus?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1


Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
Helium is the lightest noble gas and the second most abundant element (after hydrogen) in the univer...
Questions

Social Studies, 09.10.2019 19:30

Computers and Technology, 09.10.2019 19:30




Geography, 09.10.2019 19:30


English, 09.10.2019 19:30

Mathematics, 09.10.2019 19:30


Chemistry, 09.10.2019 19:30

Mathematics, 09.10.2019 19:30