

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1


Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1
You know the right answer?
Write the equilibrium constant expression, k, for the following reaction. enter the compounds in th...
Questions

Physics, 19.04.2021 20:40

Mathematics, 19.04.2021 20:40

Mathematics, 19.04.2021 20:40

Mathematics, 19.04.2021 20:40

Mathematics, 19.04.2021 20:40


English, 19.04.2021 20:40


Mathematics, 19.04.2021 20:40


Mathematics, 19.04.2021 20:40

Mathematics, 19.04.2021 20:40

History, 19.04.2021 20:40


Mathematics, 19.04.2021 20:40


Mathematics, 19.04.2021 20:40

Mathematics, 19.04.2021 20:40


Mathematics, 19.04.2021 20:40

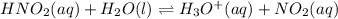
![K=[tex]K=\frac{[H_3O^+][NO_2]}{[HNO_2]}](/tpl/images/0465/0290/6446e.png)
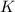 will be,
will be,![K=\frac{[H_3O^+][NO_2]}{[HNO_2]}](/tpl/images/0465/0290/adfb9.png)


