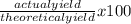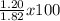Chemistry, 22.01.2020 00:31 jonmorton159
The actual yield of a product in a reaction was measured as 1.20 g. if the theoretical yield of the product for the reaction is 1.82 g, what is the percentage yield of the product?
a. 65.9%
b. 67.2%
c. 71.9%
d. 73.3%

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
The actual yield of a product in a reaction was measured as 1.20 g. if the theoretical yield of the...
Questions


Social Studies, 10.07.2019 19:00



History, 10.07.2019 19:00



Chemistry, 10.07.2019 19:00

Physics, 10.07.2019 19:00

Mathematics, 10.07.2019 19:00


Mathematics, 10.07.2019 19:00






Mathematics, 10.07.2019 19:00


Social Studies, 10.07.2019 19:00