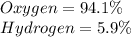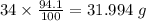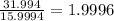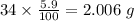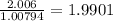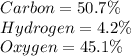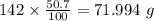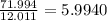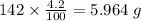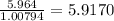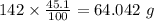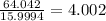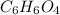Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar mass = 34g
b) 50.7% carbon, 4.2% hydrogen, and 45.1% oxygen; molar mass = 142g
(would greatly appreciated if someone could explain the process, also use the correct amount of significant digits)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3


Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar...
a) 94.1% oxygen and 5.9% hydrogen; molar...
Questions

Mathematics, 09.12.2021 03:30

Mathematics, 09.12.2021 03:30

Computers and Technology, 09.12.2021 03:30


Computers and Technology, 09.12.2021 03:30

Social Studies, 09.12.2021 03:30




Mathematics, 09.12.2021 03:30

History, 09.12.2021 03:30

Health, 09.12.2021 03:30

Geography, 09.12.2021 03:30

Social Studies, 09.12.2021 03:30





Mathematics, 09.12.2021 03:30

Computers and Technology, 09.12.2021 03:30

 molecules.
molecules.