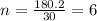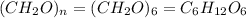Chemistry, 21.01.2020 23:31 Spoiledgirl2905
The empirical formula for a compound is ch2o, and the molar mass is 180.2 g/mol. which is the molecular formula for this compound? a) c6h12o6
b) c7h16o5
c) c8h20o4
d) c3h6o3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2


Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
The empirical formula for a compound is ch2o, and the molar mass is 180.2 g/mol. which is the molecu...
Questions

Geography, 05.06.2020 11:57



Biology, 05.06.2020 11:57



Geography, 05.06.2020 11:57

English, 05.06.2020 11:57


Mathematics, 05.06.2020 11:57

Mathematics, 05.06.2020 11:57

Mathematics, 05.06.2020 11:57

Computers and Technology, 05.06.2020 11:57



History, 05.06.2020 11:57

Mathematics, 05.06.2020 11:57


Social Studies, 05.06.2020 11:57

Mathematics, 05.06.2020 11:57

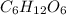
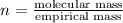
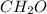 and the molar mass of compound is, 180.2 gram/mol.
and the molar mass of compound is, 180.2 gram/mol.