
Chemistry, 21.01.2020 22:31 edgartorres5123
According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.0% hcl by mass and have a density of 1.18 g/ml.
1. what is the molarity of concentrated hcl.
2. what volume of it would you need to prepare 985 ml of 1.6 m hcl?
3. what mass of sodium bicarbonate would be needed to neutralize the spill if a bottle containing 1.75 l of concentrated hcl dropped on a lab floor and broke open?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.0% hcl by ma...
Questions

English, 31.03.2021 01:00

Mathematics, 31.03.2021 01:00


Social Studies, 31.03.2021 01:00


History, 31.03.2021 01:00

Spanish, 31.03.2021 01:00

Computers and Technology, 31.03.2021 01:00




Mathematics, 31.03.2021 01:00



English, 31.03.2021 01:00


Mathematics, 31.03.2021 01:00


Arts, 31.03.2021 01:00

Spanish, 31.03.2021 01:00

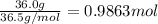
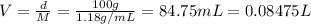
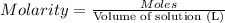
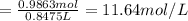
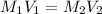 ( Dilution equation)
( Dilution equation)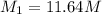
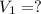
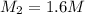
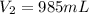
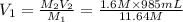
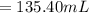
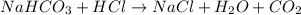
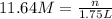
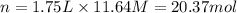
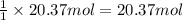 of sodium carbonate
of sodium carbonate 

