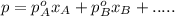Chemistry, 21.01.2020 21:31 19thomasar
Imagine a solution of two liquids in whichthe molecules interact less favorably than they do in theindividual liquids. will this solution deviate positively from, deviate negativelyfrom, or ideally follow raoult's law? a. it will deviate positively. b. it will deviate negatively. c. it will be an ideal solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
Imagine a solution of two liquids in whichthe molecules interact less favorably than they do in thei...
Questions

Chemistry, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20




Chemistry, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20

Arts, 10.12.2020 01:20


English, 10.12.2020 01:20


Mathematics, 10.12.2020 01:20

Mathematics, 10.12.2020 01:20



Mathematics, 10.12.2020 01:20