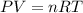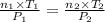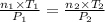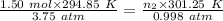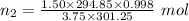Chemistry, 21.01.2020 19:31 steelemaddie20
Aglass container was initially charged with 1.50 mol of a gas sample at 3.75 atm and 21.7c. some of the gas was release as the temp was increased to 28.1c so the final pressure in the container was reduced to 0.998 atm. how many moles of the gas sample are present at the end?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1


Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
Aglass container was initially charged with 1.50 mol of a gas sample at 3.75 atm and 21.7c. some of...
Questions

Biology, 01.07.2019 10:30


Mathematics, 01.07.2019 10:30



History, 01.07.2019 10:30



Biology, 01.07.2019 10:30



Mathematics, 01.07.2019 10:30




Chemistry, 01.07.2019 10:30


Mathematics, 01.07.2019 10:30