
Chemistry, 21.01.2020 06:31 tonydeanfbg8706
Molecular iodine, i2 (g), dissociates into iodine atoms at 545k with a first order rate constant of 0.344 1/s. if you start with 0.054 m i2 at this temperature how much will remain after 5.16 s?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Molecular iodine, i2 (g), dissociates into iodine atoms at 545k with a first order rate constant of...
Questions

History, 14.02.2020 10:44

Biology, 14.02.2020 10:50

Biology, 14.02.2020 10:59


Social Studies, 14.02.2020 11:11

Mathematics, 14.02.2020 11:13


Mathematics, 14.02.2020 11:14



English, 14.02.2020 11:18

Mathematics, 14.02.2020 11:21


Mathematics, 14.02.2020 11:24

Business, 14.02.2020 11:27



English, 14.02.2020 11:33


English, 14.02.2020 11:35

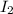 remain after 5.16 seconds.
remain after 5.16 seconds.![[A_t]=[A_0]e^{-kt}](/tpl/images/0463/7231/1ef89.png)
![[A_t]](/tpl/images/0463/7231/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0463/7231/9a686.png) is the initial concentration
is the initial concentration
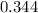 s⁻¹
s⁻¹
![[A_t]=0.054e^{-0.344\times 5.16}\ M=\frac{1\times \:0.054}{e^{1.77504}}\ M=0.00915\ M](/tpl/images/0463/7231/40d7d.png)


