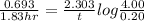Fluorine-18 (18f) is a radioactive isotope use in a variety of medical imaging procedures including positron emission tomography (pet) scans.
fluorine-18 decays by positron emission with a half-life of 1.83 hours.
(1) when 18f undergoes positron emission, the product nucleus is,
a) 18o b) 19ne c) 14n d) 17f
(2) a typical dose of 18f used for a pet scan has an activity of 4.00 millicuries. how long will it take for 95% of the 18f to decay?
a) 1.74 hrs b) 5.49 min c) 8.13 minutes d) 7.91 hrs.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1


Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
Fluorine-18 (18f) is a radioactive isotope use in a variety of medical imaging procedures including...
Questions

Biology, 20.11.2019 23:31



Mathematics, 20.11.2019 23:31



Mathematics, 20.11.2019 23:31






Mathematics, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31

History, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31

History, 20.11.2019 23:31

Social Studies, 20.11.2019 23:31

History, 20.11.2019 23:31

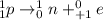
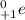 . Therefore, when a positron emission occurs then the resultant nuclei atomic number decreases by a unit mass.
. Therefore, when a positron emission occurs then the resultant nuclei atomic number decreases by a unit mass. 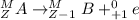
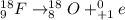
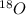 .
.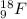 isotope is as follows.
isotope is as follows.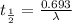
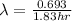
![[N]_{o}](/tpl/images/0463/6241/b711d.png) ) = 4.00 millicuries
) = 4.00 millicuries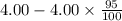
![\lambda = \frac{2.303}{t} log \frac{[N]_{o}}{[N]_{t}}](/tpl/images/0463/6241/b899d.png)