
Reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. the gas was collected by water displacement at 22 degree c. the barometric pressure in the lab was 746 mm hg. the vapor pressure of water at 22 degree c is 19.8 mm hg. use dalton's law to calculate the partial pressure of hydrogen gas in the gas-collecting tube. use the combined gas law to calculate the volume of hydrogen at stp. what is the theoretical number of moles of hydrogen that can be produced from 0.028g of mg?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1


Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
Reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. th...
Questions


Computers and Technology, 19.02.2020 05:48


Computers and Technology, 19.02.2020 05:48





World Languages, 19.02.2020 05:49

Mathematics, 19.02.2020 05:49

Mathematics, 19.02.2020 05:49









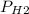 "
"
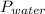
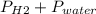
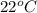 and 726.2 mm Hg and than convert it to STP conditions.
and 726.2 mm Hg and than convert it to STP conditions.
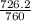
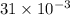 Litre
Litre
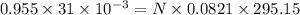
 moles
moles
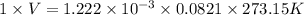
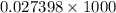 (as 1 L = 1000 ml)
(as 1 L = 1000 ml)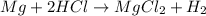
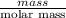
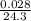
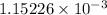 moles
moles


