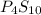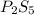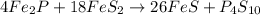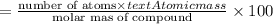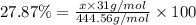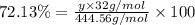Ferrophosphorus (fe2p) reacts with pyrite (fes2) producing iron(ii) sulfide and a compound that is 27.87% p and 72.13% s by mass and has a molar mass of 444.56 g/mol.
a. determine the empirical and molecular formulas of this compound.
b. empirical formula: molecular formula:
c. write a balanced chemical equation for this reaction. do not include phases.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
Ferrophosphorus (fe2p) reacts with pyrite (fes2) producing iron(ii) sulfide and a compound that is 2...
Questions

Mathematics, 21.11.2020 03:30



Biology, 21.11.2020 03:30


Social Studies, 21.11.2020 03:30

Chemistry, 21.11.2020 03:30

English, 21.11.2020 03:30



Mathematics, 21.11.2020 03:30





Mathematics, 21.11.2020 03:30

Mathematics, 21.11.2020 03:30

Mathematics, 21.11.2020 03:30