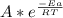Chemistry, 21.01.2020 01:31 cierramcdonald
A)if it takes 0.20 min to decompose 15% of a 0.300 m solution of nitrosyl chloride, what is k(the rate constant)? the decomposition of nitrosyl chloride is a second order reaction: nocl(> no(g)+1/2cl2(g)b)calculate the activation energy? when the temperature increases from 15c to 25c for a certain reaction, its rate constant doubles. calculate the activation energy, ea+ (remember: with r=8.31 j/mol. k, ea must be expressed in j/mol)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1
You know the right answer?
A)if it takes 0.20 min to decompose 15% of a 0.300 m solution of nitrosyl chloride, what is k(the ra...
Questions



English, 23.03.2021 18:50

Mathematics, 23.03.2021 18:50

Mathematics, 23.03.2021 18:50

Advanced Placement (AP), 23.03.2021 18:50



Mathematics, 23.03.2021 18:50






Mathematics, 23.03.2021 18:50


Mathematics, 23.03.2021 18:50

English, 23.03.2021 18:50

Mathematics, 23.03.2021 18:50