
Chemistry, 20.01.2020 21:31 maystrenko53
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. an industrial chemist studying this reaction fills a 500 ml flask with 4.8 atm of ammonia gas and 1.9 atm of oxygen gas at 33 degress celsius. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of nitrogen gas to be 0.63 atm. calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its r...
Questions

Mathematics, 01.04.2021 18:40



Mathematics, 01.04.2021 18:40

Chemistry, 01.04.2021 18:40

English, 01.04.2021 18:40




Computers and Technology, 01.04.2021 18:40

English, 01.04.2021 18:40






Computers and Technology, 01.04.2021 18:40


Mathematics, 01.04.2021 18:40

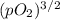 ]
]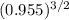 ]
]

