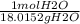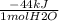Chemistry, 20.01.2020 21:31 fansofboys
Suppose that 0.88g of water condenses on a 75.0-g block of iron that is initially at 22 degrees celsius. if the heat released during condensation goes only to warming the block, what is the final temperature (in degrees celsius) of the iron block? assume a constant enthalpy of vaporization for water of 44.0 kj/mol.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
Suppose that 0.88g of water condenses on a 75.0-g block of iron that is initially at 22 degrees cels...
Questions

Mathematics, 03.05.2021 16:10


History, 03.05.2021 16:10

History, 03.05.2021 16:10



Mathematics, 03.05.2021 16:10

Mathematics, 03.05.2021 16:10



Biology, 03.05.2021 16:10

Mathematics, 03.05.2021 16:10


Mathematics, 03.05.2021 16:10

English, 03.05.2021 16:10



Advanced Placement (AP), 03.05.2021 16:10

Mathematics, 03.05.2021 16:10