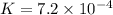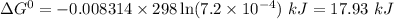Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3


Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Calculate δg° for the following reaction from the equilibrium constant at the temperature given. hf(...
Questions

Mathematics, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

History, 04.09.2020 08:01





Mathematics, 04.09.2020 08:01





English, 04.09.2020 08:01

History, 04.09.2020 08:01



Spanish, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

Mathematics, 04.09.2020 08:01

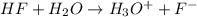
![25^oC=[25+273]K=298K](/tpl/images/0462/9739/df1f6.png)