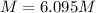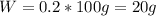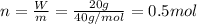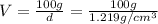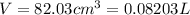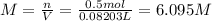Chemistry, 20.01.2020 20:31 madams4450
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molarity. (6.10 m) 2. * 10 w= amountiof solute d- density of solution motanty m= molecular mass ot solute %3d

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molar...
Questions



Computers and Technology, 22.07.2021 15:20

English, 22.07.2021 15:20

English, 22.07.2021 15:20

Engineering, 22.07.2021 15:20

English, 22.07.2021 15:20

Computers and Technology, 22.07.2021 15:20

Mathematics, 22.07.2021 15:20

History, 22.07.2021 15:20

Law, 22.07.2021 15:20

Computers and Technology, 22.07.2021 15:20



Chemistry, 22.07.2021 15:20