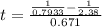Chemistry, 20.01.2020 19:31 kennedy5550
For the simple decomposition reaction: ab(g) latex: \longrightarrow⟶ a(g) + b(g), the rate = k{ab}2 ({ = [) and k = 0.67 1/mlatex: \cdot⋅s. how long (in seconds) will it take for the concentration of ab to reach one-third of its initial concentration of 2.38? enter to 2 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
For the simple decomposition reaction: ab(g) latex: \longrightarrow⟶ a(g) + b(g), the rate = k{ab}...
Questions

English, 30.08.2019 18:00

Mathematics, 30.08.2019 18:00



Biology, 30.08.2019 18:00








Physics, 30.08.2019 18:00


History, 30.08.2019 18:00

Health, 30.08.2019 18:00



Chemistry, 30.08.2019 18:00

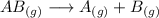 , the rate =
, the rate = ![k[AB]^2](/tpl/images/0462/8762/89597.png) and k =
and k = 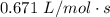 . How long (in seconds) will it take for the concentration of AB to reach one-third of its initial concentration of
. How long (in seconds) will it take for the concentration of AB to reach one-third of its initial concentration of 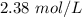 ? Enter to 2 decimal places
? Enter to 2 decimal places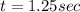
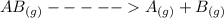
![[AB]_{o} = 2.38 mol/L](/tpl/images/0462/8762/8c514.png)
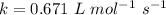
![[AB]_{t} = \frac{1}{3} [AB]_{o}](/tpl/images/0462/8762/f8015.png)
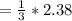
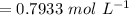 f
f![t = \frac{\frac{1}{[AB]_t} -\frac{1}{[AB]_t} }{k}](/tpl/images/0462/8762/e2e8e.png)