
Chemistry, 20.01.2020 19:31 lavardamon123
Write the formula of the conjugate base of each acid: hbr, hco3−, and ch3ch2ch2oh. be sure to answer all parts. (note: if a number has been placed as a subscript, the cursor needs to be returned to the main writing line before selecting the superscript.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
Write the formula of the conjugate base of each acid: hbr, hco3−, and ch3ch2ch2oh. be sure to answe...
Questions

Arts, 03.04.2020 11:04

Mathematics, 03.04.2020 11:05

Mathematics, 03.04.2020 11:05

Advanced Placement (AP), 03.04.2020 11:05

Mathematics, 03.04.2020 11:05

Mathematics, 03.04.2020 11:06


Law, 03.04.2020 11:06


Biology, 03.04.2020 11:08

Mathematics, 03.04.2020 11:08

Mathematics, 03.04.2020 11:08


Biology, 03.04.2020 11:10


English, 03.04.2020 11:13



Mathematics, 03.04.2020 11:15

History, 03.04.2020 11:15

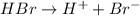
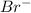 is the conjugate base of HBr.
is the conjugate base of HBr.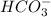
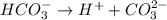
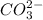 is the conjugate base of
is the conjugate base of 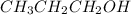
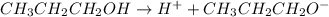
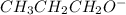 is the conjugate base of
is the conjugate base of 

