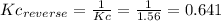Chemistry, 20.01.2020 19:31 naomijefferson22
Balance each of the following examples of heterogeneous equilibria and write each kc expression. then calculate the value of kc for the reverse reaction.
(1) al(s) + naoh(aq) + h2o(l) ⇋ na[al(oh)4](aq) + h2(g) kc for balanced reaction = 11
(2) h2o(l) + so3(g) ⇋ h2so4 (aq) kc for balanced reaction = 0.0123
(3) p4(s) + o2(g) ⇋ p4o6(s) kc for balanced reaction = 1.56

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 1
You know the right answer?
Balance each of the following examples of heterogeneous equilibria and write each kc expression. the...
Questions

Mathematics, 16.10.2021 03:00


Chemistry, 16.10.2021 03:00

Mathematics, 16.10.2021 03:00


Mathematics, 16.10.2021 03:00

Mathematics, 16.10.2021 03:00

Mathematics, 16.10.2021 03:10



Mathematics, 16.10.2021 03:10

Medicine, 16.10.2021 03:10

Biology, 16.10.2021 03:10


French, 16.10.2021 03:10




Business, 16.10.2021 03:10

 + 7 H_2(g)](/tpl/images/0462/9109/35a9e.png)
![Kc=\frac{[Na[Al(OH)_4]]^2*[H_2]^7}{[NaOH]^2}](/tpl/images/0462/9109/5fa1b.png)
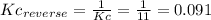
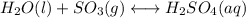
![Kc=\frac{[H_2SO_4]}{[SO_3]^2}](/tpl/images/0462/9109/da917.png)
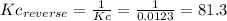
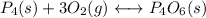
![Kc=\frac{1}{[O_2]^3}](/tpl/images/0462/9109/3ee9b.png)