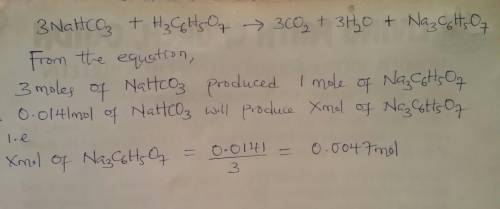when an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen carbonate (sodium bicarbonate, nahco3) and citric acid (h3c6h5o7).
3 nahco3(aq) + h3c6h5o7(aq) 3 co2(g) + 3 h2o(l) + na3c6h5o7(aq)
how many moles of na3c6h5o7 can be produced if one tablet containing 0.0141 mol of nahco3 is dissolved?
mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

You know the right answer?
when an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen car...
Questions

Mathematics, 08.04.2020 01:19



English, 08.04.2020 01:19

Biology, 08.04.2020 01:19

Mathematics, 08.04.2020 01:19

Mathematics, 08.04.2020 01:19




Mathematics, 08.04.2020 01:19





English, 08.04.2020 01:19




Mathematics, 08.04.2020 01:19