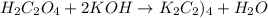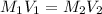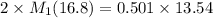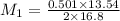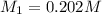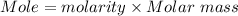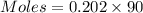Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
If 16.8 ml of the analyte h2c2o4 (oxalic acid) is titrated with 0.501 m ...
Questions

Mathematics, 23.04.2021 04:10

SAT, 23.04.2021 04:10


Mathematics, 23.04.2021 04:10

English, 23.04.2021 04:10


Mathematics, 23.04.2021 04:10



Mathematics, 23.04.2021 04:10

Mathematics, 23.04.2021 04:10

Mathematics, 23.04.2021 04:10

Mathematics, 23.04.2021 04:10

Mathematics, 23.04.2021 04:10



Mathematics, 23.04.2021 04:10